HIV Infection and AIDS
Human Immunodeficiency virus (HIV) infects one in every 200 people, infecting over 36 million people worldwide. Almost two thirds of the infections occur in eastern and southern Africa. In the US, it is estimated that more than 1.2 million people are living with HIV. As of 2019, Washington DC has one of the highest rate of infection in the US, with an estimated 1.8% rate.
After initial infection, HIV replicates and depletes CD4 T cells, leading to the deterioration of the immune system. In the absence of treatment, the disease progresses into Acquired Immunodeficiency Syndrome (AIDS). During AIDS, HIV infected individuals became susceptible to opportunistic infections by parasites (i.e. Toxoplasma spp.), Fungi (i.e. Candida spp.), or viruses (i.e. Herpes virus HHV-8, causative agent of Kaposi’s sarcoma). The decrease of the immune defenses and the increase in opportunistic infections leads to death. More than 34 million people have died of HIV/AIDS worldwide.
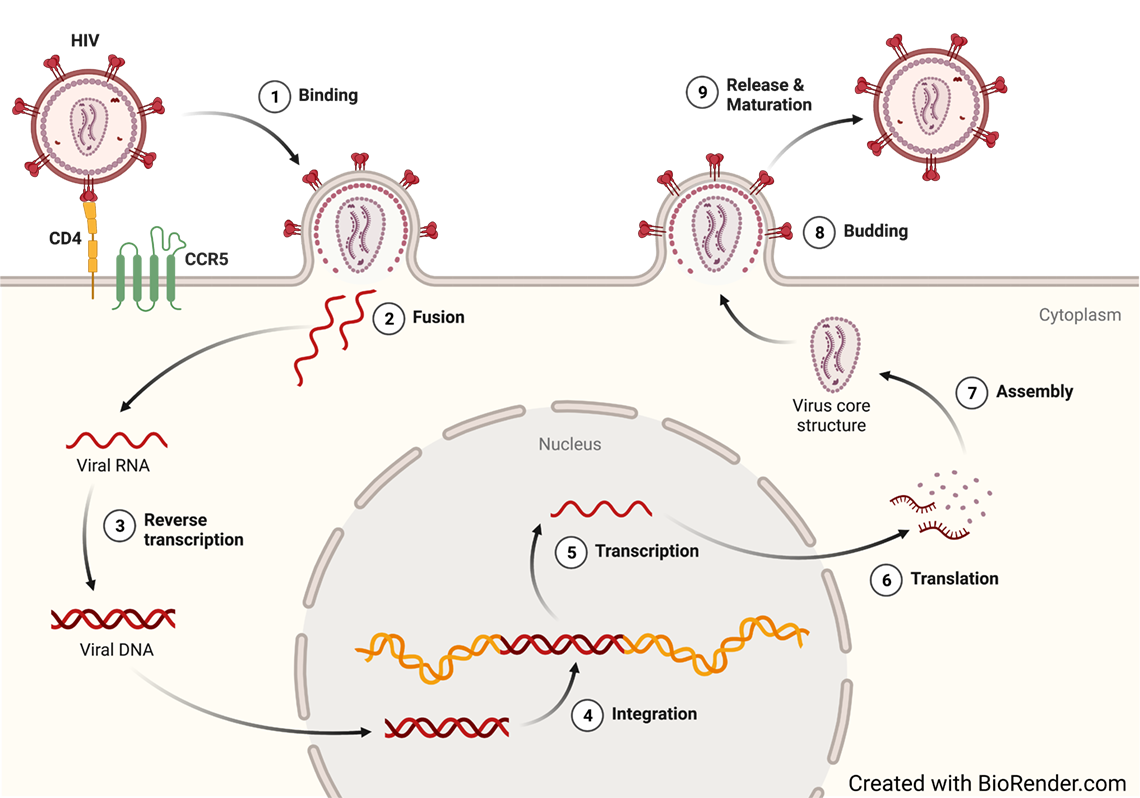
HIV Treatment
Treatment against HIV is available since the mid 90s. Current anti-retroviral therapy (ART) requires a daily administration of a combination of drugs to target different steps in HIV life cycle. This has transformed the disease from deadly to chronic.
HIV is a single-stranded, positive-sense RNA virus (ssRNA). After infection, HIV undergoes reverse transcription and transform their RNA into double-stranded DNA (dsDNA). HIV dsDNA is then integrated into the human genome. Once it is integrated, HIV can undergo transcription using the cellular machinery. Transcription leads to the production of new HIV proteins as well as its genome for the generation of new progeny virus. HIV can also remain transcriptionally silent until further stimulation. In this case, HIV become latent and invisible to the immune system as well as current therapy.
HIV Cure
In spite of its success, ART is not curative. Discontinuing therapy leads to the rebound in viremia and the progression of the disease. This is due to the present of a small number of cells in which HIV can hide from the action of the immune system and current therapies. These cells are latently infected with HIV and form a small and long-lived reservoir of HIV. Efforts are made worldwide towards designing therapies to eliminate this reservoir. Two different cure scenarios are actively being pursued. First, a sterilization cure in which the latent reservoir is completely eliminated. An example of this strategy are the “Berlin” and “London” patients. Second, a functional cure in which the infection is not completely eliminated but it is controlled by the patient’s own immune system in the absence of ART. This has been observed in some patients like the VISCONTI study. There are several different approaches, both genetic and pharmacologic, that are being explored towards achieving this goal.
The Bosque lab is seeking to understand the mechanisms that (a) control the establishment of HIV latency, (b) induce reactivation of latent viruses and (c) enhance the clearance of the latent reservoir. These studies are directed towards the development of curative strategies towards HIV-1.
Current Research Projects
Developing primary cell models of HIV latency
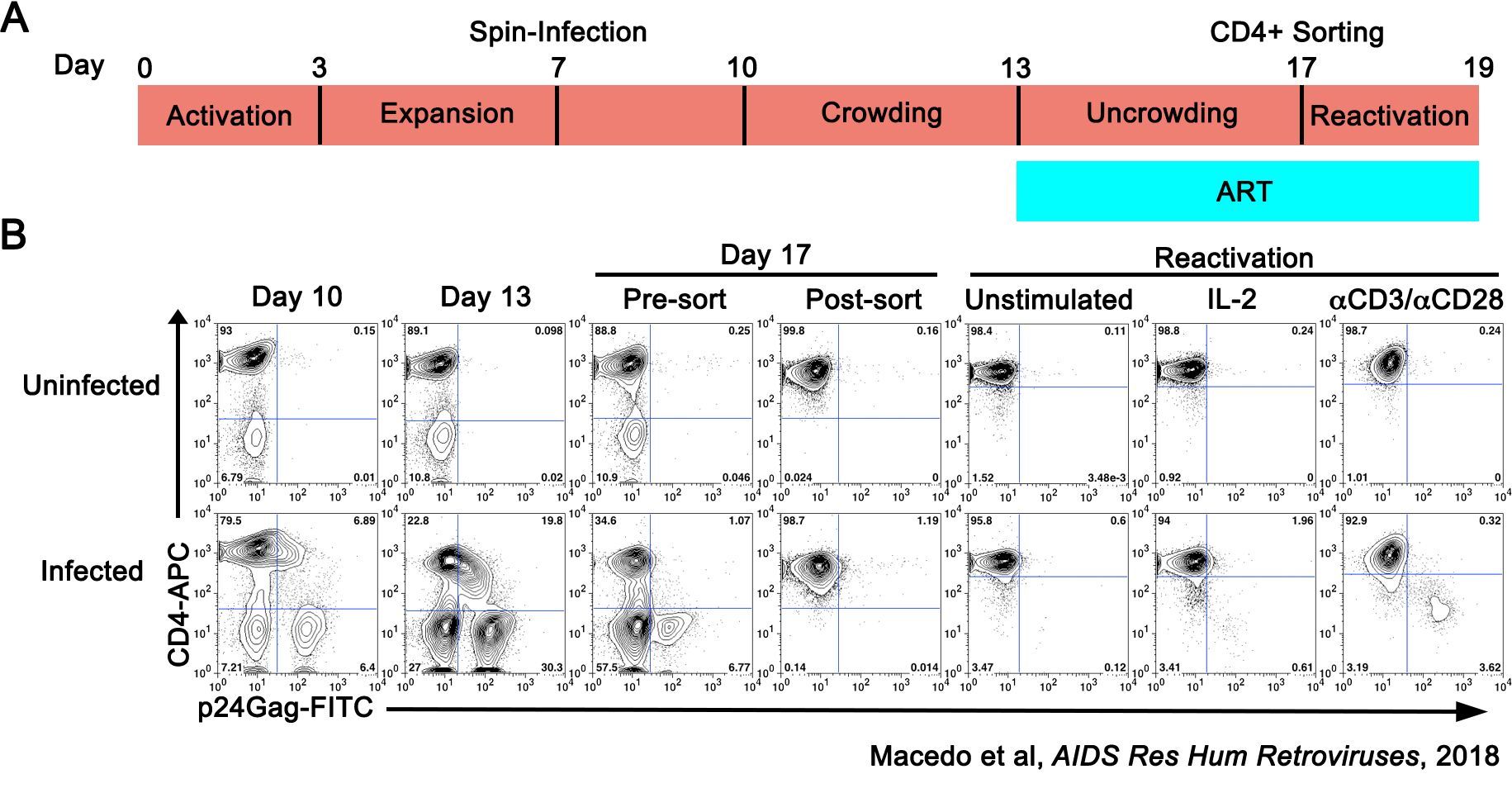
In order to develop successful HIV cure interventions, the basic mechanisms underlying latency establishment and reversal must be known. Mechanistic studies are difficult to carry out in samples from people living with HIV (PLWH) due to the availability of sample and the scarcity of these cells in vivo; thus, cell lines and primary cell models serve an important purpose in understanding the biology of HIV-1 latency. Our group has extensively characterized a primary model of latency that uses CD4T cells isolated from HIV-negative donors (Bosque and Planelles, Blood, 2009; Macedo et al, AIDS Res Hum Retroviruses, 2018; Sarabia et al, Journal of Virology, 2021). This model has been extensively used to discover and evaluate latency-reversing agents (LRAs); to perform mechanistic studies examining pathways involved in the establishment and maintenance of latency; and has several similitudes when compared with the reservoir in PLWH including similar integration patterns, clonal expansion and blocks in HIV-1 splicing. As such, this model could be used to further understand the mechanisms involved in HIV persistence in CD4 T cells as well as the pre-clinical evaluation of HIV cure strategies.
Discovering and characterizing latency-reversing agents and immunoenhancers to eradicate HIV
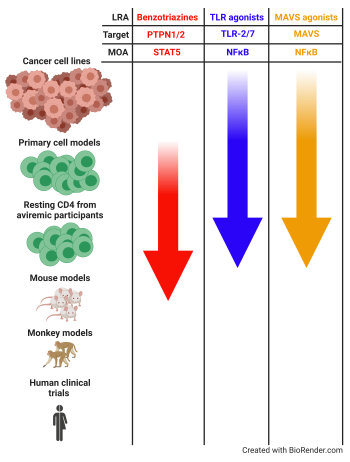
Benzotriazine derivatives to target the latent reservoir
A strategy towards HIV eradication relay in the reactivation of latent HIV-1 followed by the clearance of the reactivated cell (“shock and kill” strategies). Compounds that reactivate latent HIV are called latency-reversing agents (LRAs) and several classes of compounds are being investigated (HDAC inhibitors, PKC agonists, etc.). We have discovered and characterized two families of compounds that can reactivate and reduce latent HIV in vitro. Benzotriazole and benzotriazine derivatives have the ability to reactivate latent HIV, being benzotriazine derivatives 100 times more active than benzotriazoles (Bosque et al, Cell Reports, 2017; Sorensen et al, Antimicrobial Agents and Chemotherapy, 2020). Mechanistic studies showed that these compounds enhance STAT5 phosphorylation, increasing STAT5’s transcriptional activity and occupancy of the HIV-1 LTR. Our research is now interested in continuing preclinical studies both in vitro and in vivo.
Besides their role in HIV latency, STATs play an important role in CD8T and NK cell homeostasis and effector function. We are actively pursuing how these compounds can enhance effector function of CD8T and NK cells for their use in adoptive cell immunotherapies for HIV and cancer.
Interferons plays a crucial role in the cellular antiviral response. All the interferons mediate their signal through the activation of STAT1 and STAT2, which lead to the expression of Interferon-Stimulated Genes (ISGs). As such, we are evaluating the potential of manipulating this pathway with benzotriazine derivatives as novel antiviral drugs for HIV and other viral infections.
Developing Pathogen Recognition Receptor Agonists as Latency Reversing Agents
Pathogen recognition receptors (PRRs) are part of the innate immune system. This first line of defense recognize pathogen associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). PRRs represent a relevant target to be used for HIV-1 eradication for two reasons. First, it has been shown that several Toll-like receptors (TLR) agonists, a class of PRRs, have the ability to reactivate latent HIV-1. Among others, we have shown that TLR-2 agonists and dual TLR-2/7 agonists can reactivate latent HIV-1 in CD4 T cells (Novis et al, Retrovirology, 2013; Macedo et al, JCI Insight, 2018). Furthermore, we have discovered that RIG-I agonists can reactivate HIV-1 and that chemical manipulation of the mitochondrial antiviral signaling protein (MAVS), a downstream effector protein of RIG-I, can reactivate latent HIV in CD4 T cells (Sarabia, Novis et al, Frontiers in Immunology, 2021). Second, PAMPS increase immune activation. Therefore, targeting PRRs may act cooperatively by reactivating latent HIV and also boosting immune responses to promote viral clearance. Several PRR ligands are being investigated or in clinical trials to enhance immunity for the use in treatment of cancer, viral infection and bacterial infection (Macedo et al, Frontiers in Immunology, 2019). Our research is directed in characterizing PRR ligands and related pathways that can both reactivate latent HIV-1 in resting CD4 T cells as well as enhance immune responses.
Developing ultrasensitive methods to detect translationally competent HIV reservoirs.
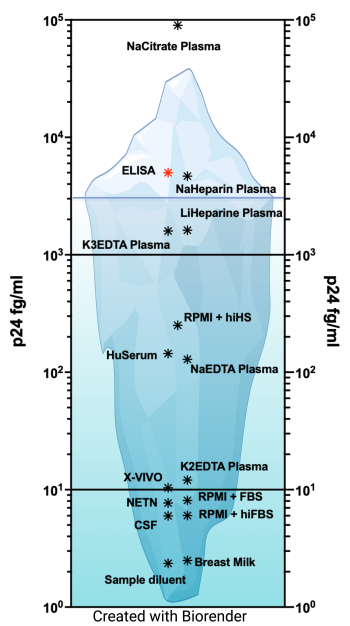
Human immunodeficiency virus-1 (HIV-1) is able to persist even in the presence of antiretroviral (ART). The development of assays to evaluate HIV persistence haa been mostly limited to the evaluation of nucleic acids, either DNA or RNA, due to their higher sensitivity compared to protein-based assays (For a review (Abdel-Mohsen et al., 2020)). These assays allow for the analysis of sequence diversity, integrated virus, intactness of the HIV genome or assessment of RNA species, including splicing forms or poly-A mRNA (Bruner et al., 2019; Butler et al., 2001; O'Doherty et al., 2002; Procopio et al., 2015; Yukl et al., 2018). However, these assays cannot evaluate the role of translationally competent viral reservoirs in HIV persistence. In the past years, several assays have been developed to evaluate expression of HIV proteins, and in particular HIV Gag. These assays use either flow cytometry (Grau-Exposito et al., 2019; Pardons et al., 2019), enzyme-linked immunosorbent assay (ELISA) using a digital ELISA (dELISA) platform (Bosque et al., 2017; Cabrera et al., 2015; Chang et al., 2013; Macedo et al., 2018; Passaes et al., 2021; Stuelke et al., 2020; Wu et al., 2021), or an enzyme-linked ImmunoSpot (ELISpot) assay called viral protein spot (VIP-Spot) (Puertas et al., 2021). We have recently developed an ultrasensitive p24 immunoassay using the Quanterix Simoa planar array technology and the SP-X imaging and analysis system (Cheng et al., 2022; Innis et al., 2021; Lambert et al., 2019; Levinger et al., 2021). This assay is performed using volumes between 25 to 50 ml in a 96-well format and it allows for the direct quantification in different matrixes without further manipulation in less than 5 hours. The limit of detection of the assay varies in function of the matrix, with some matrixes allowing detection in the low fg/ml. The levels of detection equal to a single viral particle in 50 ml or a single infected cell expressing p24 in one million cells. This planar SP-X immunoassay p24 ELISA could be an additional assay to evaluate HIV-1 cure approaches when sample volume is limited, and to study HIV-1 persistence of translationally competent reservoirs in diverse cells and/or anatomical compartments. In collaboration with the Lynch and Bethony lab at MITM, we are currently using this technology to develop an assay to detect HIV envelop expression to quantify pre-existing resistance to broadly neutralizing antibodies (bNAbs).